Preparations before calibration
Before you can start the calibration of the weighing instrument,
you should clarify a few things and get prepared.
You should find out the technical characteristics of the
weighing instrument (max weight, d value), the accuracy requirement (max error
allowed and uncertainty) and what to do if the calibration fails (adjustment).
Typically, the whole measurement range is calibrated and the
calibration is performed in the location where the instrument is being used.
Make sure you have enough weights for the calibration procedure available.
The weighing instrument should be switched on at least 30
minutes before the calibration. The temperature of the weights should be
stabilized to the same temperature where the calibration is to be done.
The weighing instrument should be at a horizontal level,
especially for small and accurate weighing instruments. Perform a few pre-tests
by placing weights close to the maximum of the range on the instrument and to
ensure it works normally.
In case the weighing instrument fails in calibration and it is
adjusted, you should make an “as found” calibration before an adjustment and an
“as left” calibration after adjustment.
In the Pharmaceutical industry
calibration of weighing balance is done on a monthly basis to check the accuracy
of the balance. While performing weighing balance calibration check the following
parameters:
·
Accuracy
·
Reproducibility
·
Eccentricity
Accuracy:
Verify the balances for
accuracy with the minimum weight (least count×100 ),5%, 20%, 50% and 90% the capacity of respective balances. Record the displayed weight in respective
monthly calibration formats.
Tolerance:
The variation if any
should be ± least count of the balance for ± 0.2 % of the certified value of the standard weight used whenever is higher and for analytical balance, the
variation should be ± least count of the balance or ± 0.1 % of the certificate
value of standard weight used whenever it is higher.
Reproducibility:
Check the
Reproducibility by using the minimum standard weight of balance capacity.
place the weight in the middle of the weighing pen and observe the displayed
value. repeat the procedure 9 times for the standard weight and record
the reading. Calculate % RSD for both the standard weight by the following
formula.
where x= individual
value
x̄= mean of the
individual value
n= number of values.
RSD= SD*100/ X
Acceptance criteria: %RSD not more than 2.0
%. Record the observations in monthly calibration record as per “monthly calibration
record of weighing balance”.
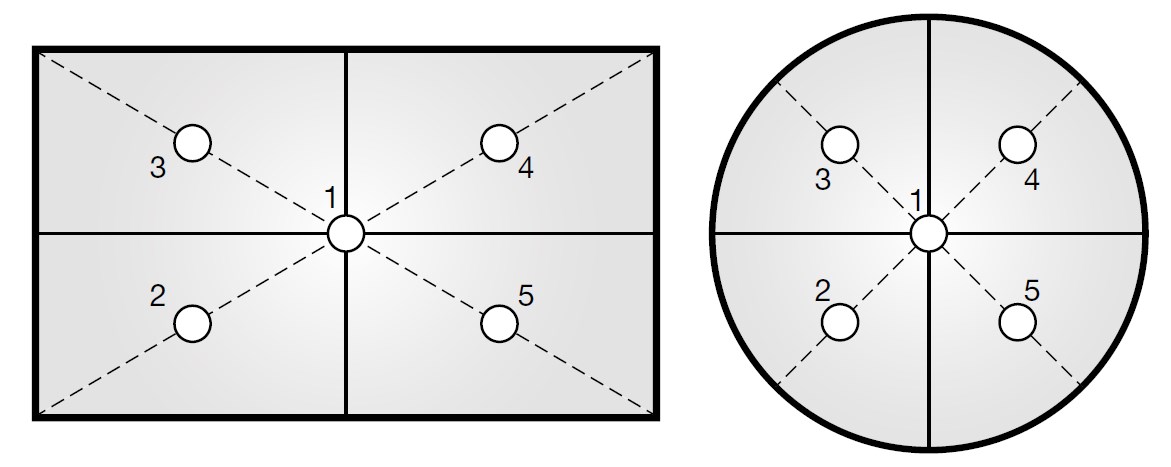
Eccentricity:
Take the minimum weight and
keep at on specified position as shown below calculate %RSD by following the formula is specified above.
Acceptance criteria:
% RSD not more than 2.0
%. Record the details of calibration in labels with Marker Pen. write the
identification number of the standard weight used during verification and
calibration in the designated place of verification and calibration records.
Calibration record shall be verified by
a quality assurance by
putting “reviewed by quality assurance” stamp along with initials and
date. calibration of analytical balance in the quality control department shall
be done as per their respective SOP.
Calibration of balances by external agency:
Internal calibration
(software calibration) of weighing balances shall be done by external agencies.
calibration certificate received from an external agency shall be reviewed
comprising the detail of balance and calibration summary.
Action to be taken if out of
calibration:
If the balance is out
of calibration to refer sop “handling of out of calibration” and rise
deviation as per SOP “handling of deviation” and a fix the label “out of
calibration” as per SOP (status labeling) and inform to Department Head of
Investigation.
Raise maintenance memo
and inform the engineering department for rectification action. if required
take a balance of configuration from another section and department for
carrying out the weighing activity. In Such situations, carry out Daily
verification of transferred balance and used for Weighing. if the parameter of
Daily verification does not meet with the specification, monthly calibration
should be done. Once rectification of the balance is done, calibrate the
balance and record the same in respective calibration records. the balance
shall be released for further use only on satisfactory closure of the deviation
with detail result of Investigation.
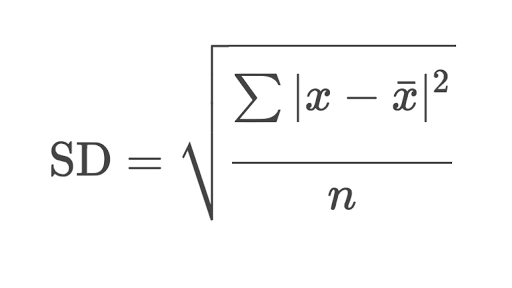
Hello Dear..
ReplyDeleteI appreciate your Informative post and It's very helpful.thanks for sharing Keep it up!
Weighing Balance | Testing Instruments | Bursting Strength Tester Digital
Thanks for appreciation!!
ReplyDelete